660
shares
There are six main characteristics of Ni-MH batteries: charging and discharging characteristics, self-discharging characteristics and long-term storage characteristics, and comprehensive cycle life characteristics and safety characteristics. They all depend on the structure of the battery, which is reflected in the environment. The remarkable feature is that they are greatly influenced by temperature and current.
1. Charging characteristics: When the charging current of Ni-MH batteries increases and/or the charging temperature decreases, the charging voltage of Ni-MH batteries increases. Generally, constant current charging is adopted at ambient temperature between 0 40 C, and higher charging efficiency can be obtained at 10 30 C.
If batteries are often charged in a high or low-temperature environment, the performance of power batteries will be reduced. For fast charging above 0.3C, charging control measures are essential. Repeated overcharging will also reduce the performance of the battery, so the protection measures of high and low temperature and high current charging must be in place.
2. Discharge characteristics: The discharge platform of Ni-MH battery is 1.2V. The higher the current, the lower the temperature, the lower the discharge voltage and efficiency of the battery, and the maximum continuous discharge current of the battery is 3C.
The discharge cut-off voltage of batteries is generally set at 0.9V, and the IEC standard charging and discharging mode is set at 1.0V. Because the discharge cut-off voltage of nickel-hydrogen batteries can be regarded as a voltage range of 0.9V~1.0V, some batteries can also provide a stable current under 1.0V, while the discharge cut-off voltage of nickel-hydrogen batteries can be regarded as a voltage range of 0.9V~1.0V. It can be marked as 0.8V below. In general, if the cut-off voltage is set too high, the battery capacity can not be fully utilized, otherwise, it is easy to cause battery over-discharge.
3. Self-discharge characteristics: refers to the phenomenon of capacity loss when the battery is fully stored in an open circuit. Self-discharge characteristics are mainly affected by environmental temperature. The higher the temperature, the greater the capacity loss after storage.
4. Long-term storage characteristics: mainly refers to the capacity recovery of Ni-MH batteries. After a long period of storage (e.g. one year), the capacity of the battery may be smaller than that before storage, but after several charging and discharging cycles, the battery can recover to its pre-storage capacity.
5. Cyclic life characteristics: The cycle life of Ni-MH batteries is affected by the charging and discharging system, temperature and operation method. According to the IEC standard, a full charge-discharge cycle is the charge cycle of Ni-MH batteries. Multiple charge cycles constitute cycle life. The charge-discharge cycle of Ni-MH batteries can exceed 500 cycles.
6. Safety: The safety performance of Ni-MH batteries is better in battery design, which is related to the material used and the structure of Ni-MH batteries. In the use process, if the improper use of the battery causes overcharge, over-discharge and short circuit, and causes the internal pressure of the battery to rise, then a recoverable safety valve will open, which can reduce the internal pressure and prevent the battery from exploding.

Nickel-hydrogen battery is a kind of battery with good performance. Nickel-hydrogen batteries are divided into high-voltage nickel-hydrogen batteries and low-voltage nickel-hydrogen batteries. The positive active material of nickel-hydrogen battery is Ni (OH) 2 (called NiO electrode), the negative active material is metal hydride, also known as hydrogen storage alloy (called hydrogen storage electrode), and the electrolyte is 6mol/L potassium hydroxide solution. As an important direction of hydrogen energy application, nickel-hydrogen batteries have attracted more and more attention.
In recent years, more and more attention has been paid to the development and utilization of hydrogen energy due to the fact that fossil fuels are less and less used on a large scale by human beings. As an important direction of hydrogen energy application, nickel-hydrogen batteries have attracted more and more attention. Although nickel-hydrogen batteries are indeed good performance batteries, space nickel-hydrogen batteries are high-pressure nickel-hydrogen batteries (hydrogen pressure can reach 3.92 MPa, or 40kg/cm2). Such high-pressure hydrogen storage in thin-walled containers is easy to explode, and nickel-hydrogen batteries need precious metals as catalysts to make them expensive. This is difficult to accept for civilian use. Therefore, low-voltage Ni-MH batteries for civilian use have been explored abroad since the 1970s. Nickel-hydrogen batteries are divided into high-voltage nickel-hydrogen batteries and low-voltage nickel-hydrogen batteries. High-voltage nickel-hydrogen batteries were first developed by M. Klein and J. F. Stockel in the early 1970s. The trend of replacing nickel-cadmium batteries with nickel-hydrogen batteries and applying them to various satellites has been formed.
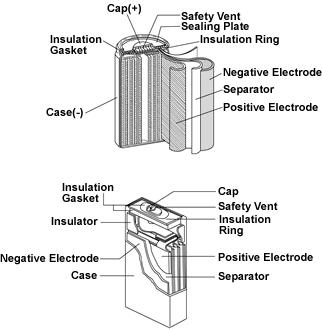
The positive active material of nickel-hydrogen battery is Ni (OH) 2 (called NiO electrode), the negative active material is metal hydride, also known as hydrogen storage alloy (called hydrogen storage electrode), and the electrolyte is 6mol/L potassium hydroxide solution. The electrode material of active material is mainly composed of sintered, pulping, nickel foam, nickel and infiltration. The electrodes produced by different processes have great differences in capacity and discharge performance. The electric pool is generally produced according to different conditions. Most of the civilian batteries such as communications are made of the negative electrode and positive nickel foam. The charge-discharge chemical reactions are as follows [1]:
Positive pole: Ni(OH)2+OH-=NiOOH+H2O+e-
Negative pole: M+H_2+e-=MHab+OH-
Total reaction: Ni(OH)2+M=NiOOH+MH
Note: M: Hydrogen alloy; Hab: Adsorption of hydrogen; The process of reaction from left to right is the charging process; The process of reaction from right to left is the discharging process.
NiOOH and OH-react to form NiOOH and H2O, and release e-to form MH and OH-together. The total reaction is Ni(OH)2 and M to form NiOOH and hydrogen storage alloy. On the contrary, MHab releases H+, H+ and OH-to form H and e-, NiOH, H and e-to regenerate Ni(OH)2 and OH-. The standard electromotive force of the battery is 1.319V.
Nickel-hydrogen batteries are divided into high-voltage nickel-hydrogen batteries and low-voltage nickel-hydrogen batteries.
Low-voltage nickel-hydrogen batteries have the following characteristics: (1) battery voltage is 1.2-1.3V, which is equivalent to that of nickel-cadmium batteries; (2) high energy density, which is more than 1.5 times that of nickel-cadmium batteries; (3) fast charge and discharge, good low-temperature performance; (4) sealed, strong overcharge and discharge resistance; (5) dendrite-free crystal formation, which can prevent batteries. Internal short circuit; (6) Safety and reliability, no pollution to the environment, no memory effect, etc. [1]
High voltage nickel-hydrogen batteries have the following characteristics: (1) high reliability. It has good over-discharge and over-charge protection can withstand high charge-discharge rate and no dendrite formation. It has good bit-rate characteristics. Its mass-specific capacity is 60A. h/kg, which is five times as large as that of nickel-cadmium batteries. (2) Long cycle life, up to thousands of times. (3) Fully sealed, less maintenance. (4) The cryogenic performance is excellent, and the capacity does not change significantly at - 10 C.
Nickel-hydrogen batteries should be maintained in use.
(1) Avoid overcharging during use. In the cycle life, the use of the process should not be overcharged, which is because overcharging easily makes the positive and negative electrodes expand, resulting in active substances falling off and diaphragm damage, conductive network damage and battery oh mic polarization become larger and so on.
(2) Preventing deterioration of electrolyte. During the cycle life of Ni-MH batteries, hydrogen evolution should be inhibited.
(3) Storage of Ni-MH batteries. The storage of Ni-MH batteries should be fully charged. If the batteries are not stored in the battery for a long time, the function of negative hydrogen storage alloy will be weakened and the battery life will be shortened.
(4) Charge when the electricity is exhausted. Nickel-hydrogen battery and nickel-cadmium batteries have the same "memory effect", that is, if the battery is recharged repeatedly in the state of residual electric energy during discharge, the battery will soon be out of use.
660
shares
contact us

GUANGZHOU GEILIENERGY ELECTRONIC CO., LTD.
We provide customers with quality products and provide high-quality services
If you would like to leave us a comment please go to contact us

